Sepsis
Sepsis is a life-threatening medical condition caused by an intense immune response to infection. The natural chemicals released in the bloodstream to combat infection trigger system-wide inflammation, which can lead to multi-organ failure and death. One of the prominent issues with sepsis is correctly diagnosing and treating the source of the infection in a timely manner. Standard treatment for sepsis involves administering broad-spectrum antibiotics. However, issues with this manner of treatment include the lack of specificity with the antibiotics and a requirement for extremely high doses in order to render the treatment effective.
Due to the nonspecific symptoms of sepsis that overlap with a multitude of other conditions making sepsis difficult to diagnose, it has been estimated that the incidence of sepsis is severely underreported. Even so, current estimates suggest that sepsis occurs in approximately 2% of all hospitalizations and affects around 750,000 people a year in the United States alone. Additionally, sepsis is the second leading cause of death in non-coronary intensive care units (ICU) and the tenth leading cause of death nationwide. Consequently, the total cost of care per patient with sepsis reaches up to $50,000 — an economic burden costing the U.S. $17 billion a year.
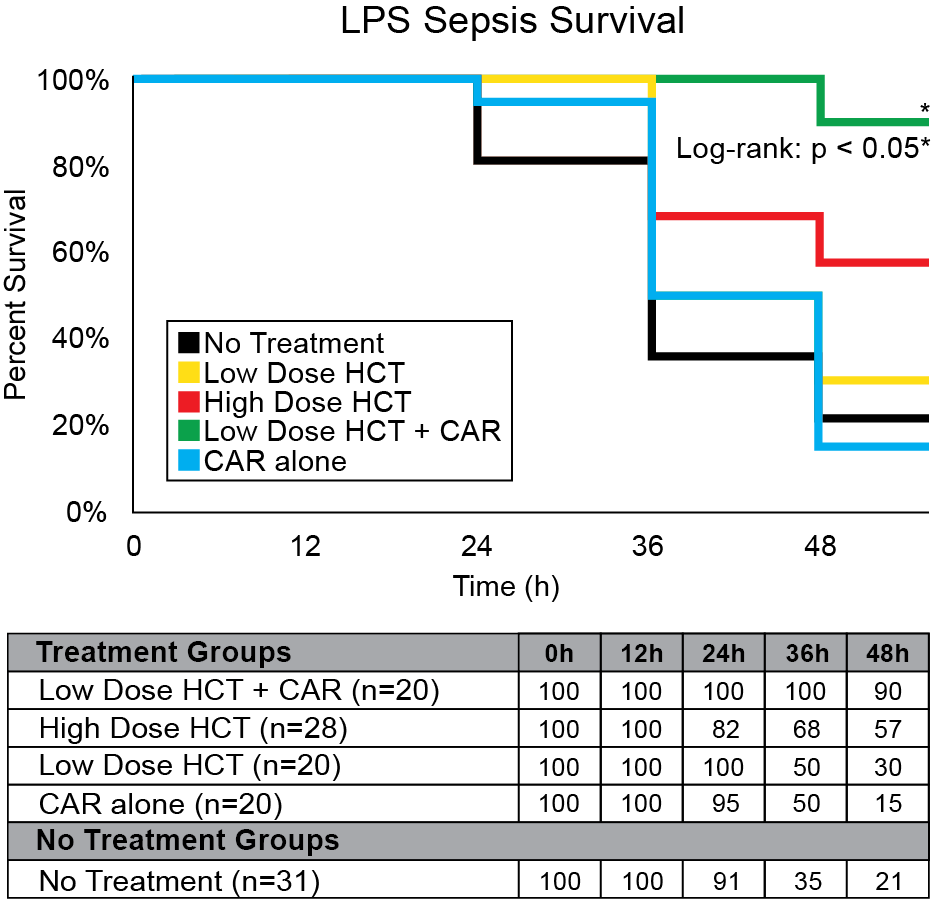
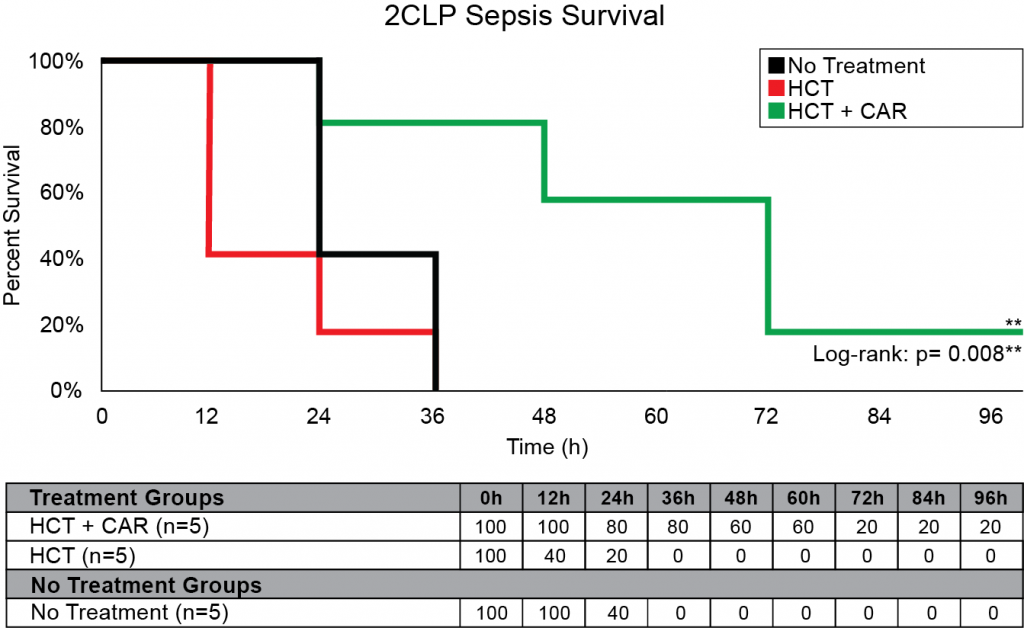
Our company discovered that CARSKNKDC (CAR), a synthetic cyclic peptide, selectively accumulates at sites of septic-damaged tissue and enhances the therapeutic effects of low-dose corticosteroids to improve survival rates in two animal models of sepsis. Specifically, we found that: (1) The mechanism of CAR homing is enhanced selective macropinocytosis at sites of damaged endothelium that enhance cellular uptake of co-administered drugs; (2) CAR selectively targets and penetrates sites of septic-injury in the lung, liver, and kidney, but does not home to healthy tissues; (3) co-administration of CAR with low dose corticosteroids restores glycocalyx damage and endothelial injuries in the kidney, lung, and liver to normal microscopic structure; (4) co-administration of CAR with low dose corticosteroids increases survival in LPS-septic mice to 90% compared to 30% for septic mice treated with low-dose corticosteroids alone, and 21% in untreated septic mice (Figure 1) The addition of CAR to low-dose cortiocsteroids also also significantly improves survival in a 2CLP rat model of sepsis (Figure 2).
Suzuki K, Okada H, Takada C, Oda K, Yoshida T, Mann D, Komatsu M, Ogura S. Hydrocortisone Therapy with CAR peptide protects the injured endothelial glycocalyx in sepsis. Critical Care Medicine. 2016 December; 44(12):407.
Yehya, N, Weiss, L, Mann D. Improved Survival After Surgical Sepsis in Rats Using a Novel Vascular Homing Peptide to Target Endothelial Delivery of Hydrocortisone. ATS 2020. American Thoracic Society 2020 International Conference; 2020 May; Philadelphia, PA, USA. ajrccmconference.2020.201.1_MeetingAbstracts.A7856
The cortiocosteroid dexamethasone was the first drug to show improved survival of COVID-19 patients.27-28 Based on this data and the recent recommendation from multiple institutes including the NIH (National Institutes of Health), IDSA (Infectious Disease Society of America), SCCM (Society of Critical Care Medicine) and ESICM (European Society of Intensive Care Medicine) for low-dose corticosteroid treatment in COVID-19 patients,14 corticosteroids show strong promise as a candidate for COVID-19 treatment development.
In response to the urgent public health need for COVID-19 therapies that improve survival, VBS is fast-tracking CAR peptide as an adjuvant therapy to improve targeting of corticosteroids such as dexamethasone. Ultimately, we seek to develop a novel therapeutic solution that can be realistically implemented at the bedside to improve survival of critically ill COVID-19 patients.
CAR represents a new paradigm in sepsis treatment that would address the issues of both high dosing and lack of specificity in septic patients, as well as aid in reducing the high mortality rate of U.S. patients affected by sepsis every year. Preclinical animal studies have shown promising results that demonstrate CAR’s ability to dramatically improve survival rates when co-administered with currently available existing drugs at highly reduced doses. As there are no existing treatments for sepsis that use a peptide to enhance previously approved therapies, CAR represents a highly targeted, innovative therapy designed to improve survival rates in septic patients without a need for high therapeutic dosing.
Next Steps:
- Learn more about the CAR peptide works
- Applications for the CAR peptide
- CAR Peptide Scientific Articles
- For inquiries about CAR peptide and other VBS pharmaceutical products,
please contact us at pharmaceuticals@vascularbiosciences.com
DISCLAIMER: The material provided on this site is strictly for informational purposes. Do not use this site as a substitute for medical care or medical advice. Please consult with your physician or other medical care provider regarding any medical questions you may have.

