VBS Response to COVID-19
SARS-COV-2 has infected millions of people globally and the mortality rate is estimated to be 1%.1 In response to this public health crisis, VBS is harnessing all of our products and research to meet this urgent threat.
The most common complication of COVID-19 is sepsis and the Global Sepsis Alliance has stated that SARS-CoV-2 causes sepsis.2 Sepsis is a medical emergency involving life-threatening organ dysfunction due to a dysregulated response to infection. In turn, it can lead to septic shock, pneumonitis, acute respiratory distress syndrome (ARDS), organ failure, and death. The effects of COVID-19 on the respiratory system are well-known, with most people requiring hospital admission developing pneumonia of varying severity. Similar to severe acute respiratory syndrome coronavirus (SARS-CoV), SARS-CoV-2 infection causes clusters of severe respiratory illness. However, multiple studies have shown that all other organ systems can be affected, most likely due to a combination of direct viral invasion and sepsis.3-14 A recent study of patients hospitalized due to COVID-19 showed that 42% of survivors and 100% of non-survivors with developed sepsis.15
Since no therapy has been shown to be sufficiently effective against the SARS-CoV-2 itself, therapies to improve COVID-19 survival should include treatment for sepsis. Despite decades of research, novel therapies to facilitate precision medicine for sepsis beyond resuscitation and infectious source control remain elusive. Use of low doses of corticosteroids, such as hydrocortisone and dexamethasone, have been investigated for treating sepsis and SARS related respiraory disease and have been shown to slightly decrease the morality rate and time spent in the ICU and on the ventilator.16-25 Additionally, there is no data showing any harmful effects of using corticosteroids for COVID-19.26
Our company discovered that CARSKNKDC (CAR), a synthetic cyclic peptide, selectively accumulates at sites of septic-damaged tissue and enhances the therapeutic effects of low-dose corticosteroids to improve survival rates in two animal models of sepsis. Specifically, we found that: (1) The mechanism of CAR homing is enhanced selective macropinocytosis at sites of damaged endothelium that enhance cellular uptake of co-administered drugs; (2) CAR selectively targets and penetrates sites of septic-injury in the lung, liver, and kidney, but does not home to healthy tissues; (3) co-administration of CAR with low dose corticosteroids restores glycocalyx damage and endothelial injuries in the kidney, lung, and liver to normal microscopic structure; (4) co-administration of CAR with low dose corticosteroids increases survival in LPS-septic mice to 90% compared to 30% for septic mice treated with low-dose corticosteroids alone, and 21% in untreated septic mice (Figure 1) The addition of CAR to low-dose cortiocsteroids also also significantly improves survival in a 2CLP rat model of sepsis (Figure 2).
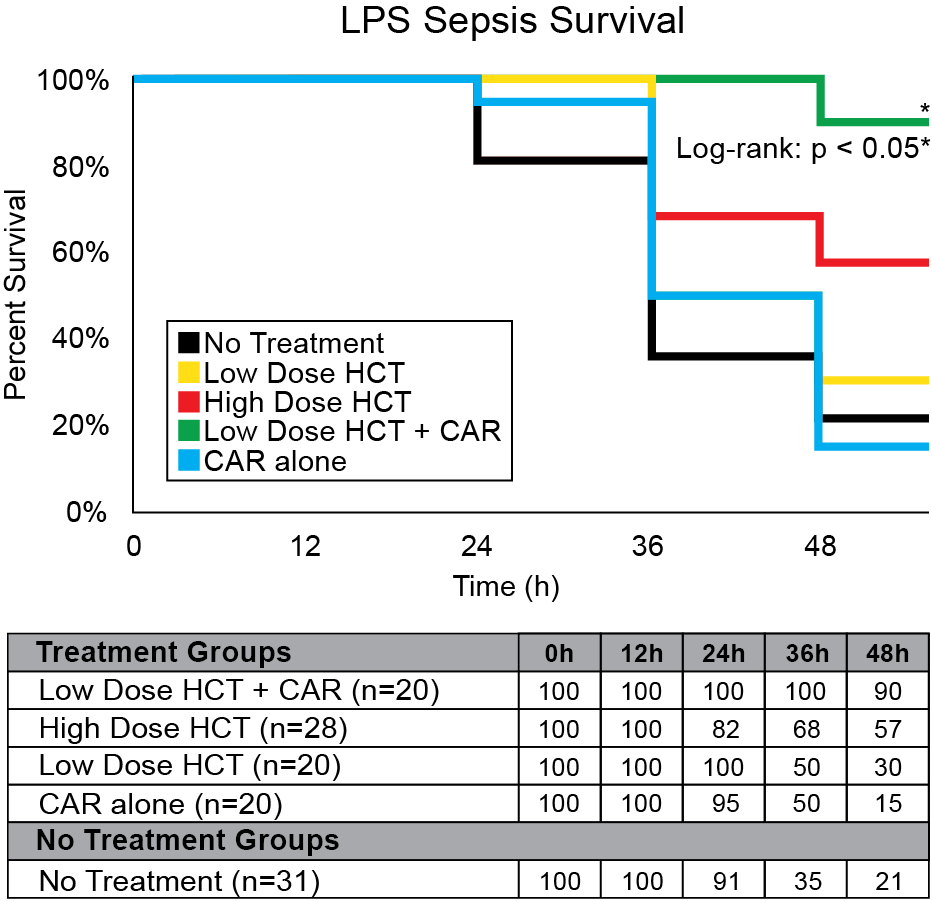
Figure 1: LPS sepsis survival rates with CAR + hydrocortisone (HCT) treatment. 48 hours after LPS administration, Group CAR + Low-HCT showed the best survival rate compared with Group No Treatment (90% vs. 21%, < 0.05) and a higher survival rate than Group High HCT. There was no significant survival difference between Groups CAR alone, Low-HCT, and no treatment.
Suzuki K, Okada H, Takada C, Oda K, Yoshida T, Mann D, Komatsu M, Ogura S. Hydrocortisone Therapy with CAR peptide protects the injured endothelial glycocalyx in sepsis. Critical Care Medicine. 2016 December; 44(12):407.
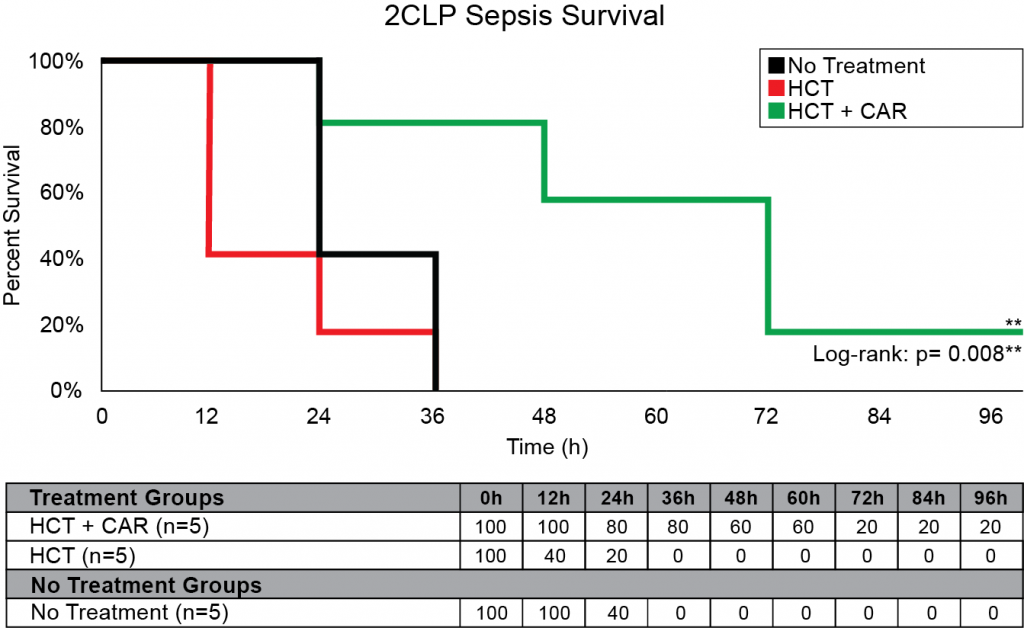
Figure 2: 2CLP Sepsis Survival with CAR peptide. 15 male Sprague Dawley rats were tested using a 2CLP model of Sepsis and monitored for 96 hours. CAR was given at a dosage of 3 mg/kg and HCT at a dosage of 0.2 mg/kg with time points at 8, 20, 32, 44, 56, and 68 hours.
Yehya, N, Weiss, L, Mann D. Improved Survival After Surgical Sepsis in Rats Using a Novel Vascular Homing Peptide to Target Endothelial Delivery of Hydrocortisone. ATS 2020. American Thoracic Society 2020 International Conference; 2020 May; Philadelphia, PA, USA. ajrccmconference.2020.201.1_MeetingAbstracts.A7856
The cortiocosteroid dexamethasone was the first drug to show improved survival of COVID-19 patients.27-28 Based on this data and the recent recommendation from multiple institutes including the NIH (National Institutes of Health), IDSA (Infectious Disease Society of America), SCCM (Society of Critical Care Medicine) and ESICM (European Society of Intensive Care Medicine) for low-dose corticosteroid treatment in COVID-19 patients,14 corticosteroids show strong promise as a candidate for COVID-19 treatment development.
In response to the urgent public health need for COVID-19 therapies that improve survival, VBS is fast-tracking CAR peptide as an adjuvant therapy to improve targeting of corticosteroids such as dexamethasone. Ultimately, we seek to develop a novel therapeutic solution that can be realistically implemented at the bedside to improve survival of critically ill COVID-19 patients.
1. Infection fatality rate of COVID-19 inferred from seroprevalence data October 14, 2020. Available at https://www.who.int/bulletin/volumes/99/1/20-265892/en/
2. Zick, M. Can COVID-19 Cause Sepsis? Explaining the Relationship Between the Coronavirus Disease and Sepsis. Global Sepsis Alliance. (2020). Available at: https://www.global-sepsis-alliance.org/news/2020/4/7/update-can-covid-19-cause-sepsis-explaining-the-relationship-between-the-coronavirus-disease-and-sepsis-cvd-novel-coronavirus
3. Bhatraju, P. K. et al. Covid-19 in Critically Ill Patients in the Seattle Region – Case Series. N. Engl. J. Med. 382, 2012–2022 (2020).
4. Arentz, M. et al. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA (2020) doi:10.1001/jama.2020.4326. Available at: https://www.nejm.org/doi/full/10.1056/nejmoa2004500
5. Chen, N. et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395, 507–513 (2020). Available at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30211-7/fulltext
6. Guan, W.-J. et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med.382, 1708–1720 (2020). Available at: https://www.nejm.org/doi/full/10.1056/nejmoa2002032
7. Shi, H. et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 20, 425–434 (2020). Available at: https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(20)30086-4/fulltext
8. Xu, L., Liu, J., Lu, M., Yang, D. & Zheng, X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 40, 998–1004 (2020). Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/liv.14435
9. Guo, T. et al. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol (2020) doi:10.1001/jamacardio.2020.1017. Available at: https://jamanetwork.com/journals/jamacardiology/fullarticle/2763845
10. Peerapornratana, S., Manrique-Caballero, C. L., Gómez, H. & Kellum, J. A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 96, 1083–1099 (2019). Available at: https://www.sciencedirect.com/science/article/abs/pii/S0085253819306015?via%3Dihub
11. Uchino, S. et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818 (2005). Available at: https://jamanetwork.com/journals/jama/fullarticle/201386
12. Doi, K., Leelahavanichkul, A., Yuen, P. S. T. & Star, R. A. Animal models of sepsis and sepsis-induced kidney injury. J. Clin. Invest. 119, 2868–2878 (2009). Available at: https://www.jci.org/articles/view/39421
13. Diao, B. et al. Human Kidney is a Target for Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection. Infectious Diseases (except HIV/AIDS) (2020) doi:10.1101/2020.03.04.20031120. Available at: https://www.medrxiv.org/content/10.1101/2020.03.04.20031120v4
14. Richardson, S. et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA vol. 323 2052 (2020). Available at: https://jamanetwork.com/journals/jama/fullarticle/2765184
15. Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020). Available at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30566-3/fulltext
16. Ince, C. et al. THE ENDOTHELIUM IN SEPSIS. Shock 45, 259–270 (2016). Available at: https://journals.lww.com/shockjournal/Fulltext/2016/03000/The_Endothelium_in_Sepsis.5.aspx
17. Minneci, P. C., Deans, K. J., Eichacker, P. Q. & Natanson, C. The effects of steroids during sepsis depend on dose and severity of illness: an updated meta-analysis. Clin. Microbiol. Infect. 15, 308–318 (2009). Available at: https://www.sciencedirect.com/science/article/pii/S1198743X14603918?via%3Dihub
18. Marik, P. E. Glucocorticosteroids as Adjunctive Therapy for Acute Respiratory Distress Syndrome and Sepsis? Yes, But Not as Monotherapy. Critical care medicine vol. 45 910–911 (2017). Available at: https://journals.lww.com/ccmjournal/FullText/2017/05000/Glucocorticosteroids_as_Adjunctive_Therapy_for.23.aspx
19. Keh, D. et al. Effect of Hydrocortisone on Development of Shock Among Patients With Severe Sepsis: The HYPRESS Randomized Clinical Trial. JAMA 316, 1775–1785 (2016). Available at: https://jamanetwork.com/journals/jama/fullarticle/2565176
20. Venkatesh, B. et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N. Engl. J. Med. 378, 797–808 (2018). Available at: https://www.nejm.org/doi/full/10.1056/NEJMoa1705835
21. Annane, D. et al. Corticosteroids for treating sepsis. Cochrane Database Syst. Rev. CD002243 (2015). Available at: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD002243.pub3/full
22. Schumer, W. Steroids in the treatment of clinical septic shock. Ann. Surg. 184, 333–341 (1976). Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1344393/
23. Chen, R.-C. et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 129, 1441–1452 (2006). Available at: https://www.sciencedirect.com/science/article/pii/S0012369215507459
24. Li, H. et al. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respi. Viruses 11, 345–354 (2017). Available at: https://onlinelibrary.wiley.com/doi/full/10.1111/irv.12456
25. Siemieniuk, R. A. C. et al. Corticosteroid Therapy for Patients Hospitalized With Community-Acquired Pneumonia: A Systematic Review and Meta-analysis. Ann. Intern. Med. 163, 519–528 (2015). Available at: https://www.acpjournals.org/doi/10.7326/M15-0715
25. Shang, L., Zhao, J., Hu, Y., Du, R. & Cao, B. On the use of corticosteroids for 2019-nCoV pneumonia. The Lancet vol. 395 683–684 (2020). Available at: https://www.sciencedirect.com/science/article/pii/S0140673620303615?via%3Dihub
26. Suzuki K, Okada H, Takada C, Oda K, Yoshida T, Mann D, Komatsu M, Ogura S. Hydrocortisone Therapy with CAR peptide protects the injured endothelial glycocalyx in sepsis. Critical Care Medicine. 2016 December; 44(12):407.
27. Yehya, N, Weiss, L, Mann D. Improved Survival After Surgical Sepsis in Rats Using a Novel Vascular Homing Peptide to Target Endothelial Delivery of Hydrocortisone. ATS 2020. American Thoracic Society 2020 International Conference; 2020 May; Philadelphia, PA, USA. ajrccmconference.2020.201.1_MeetingAbstracts.A7856
DISCLAIMER: The material provided on this site is strictly for informational purposes. Do not use this site as a substitute for medical care or medical advice. Please consult with your physician or other medical care provider regarding any medical questions you may have.