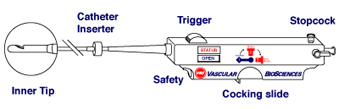
Endoarterial Biopsy Catheter
The endoarterial biopsy (EABx) catheter: A minimally invasive cardiovascular catheter designed to exclusively biopsy the inner layers of arteries.
VBS Interventional has developed the endoarterial biopsy catheter for the study, diagnosis, monitoring, and treatment of vascular-based diseases.
The endoarterial biopsy catheter will provide physicians with the ability to percutaneously obtain biopsy specimens from the inner layers of the arterial wall.
Arterial biopsies can be analyzed using histopathological, genomic, and proteomic methods in order to describe current vascular disease status; they also provide important information about eventual disease progression as well as enable the design of individualized, effective therapies.
The catheter is compatible with the existing cardiac catheterization environment. Endoarterial biopsies can eventually be performed on an outpatient basis.
The device is intended for single use only. It can be used to obtain multiple biopsy samples from multiple arterial locations during a single procedure and is then disposed of.
Learn More about:
- The endoarterial biopsy procedure
- The endoarterial biopsy sequence
- The endoarterial biopsy sample
- Endoarterial biopsy single use components
- Endoarterial biopsy catheter kit
- For more about the VBS endoarterial biopsy catheter, please contact us at interventional@vascularbiosciences.com
WARNING: Investigational Device. Limited by Federal law to investigational use. This device should be used only by physicians with a thorough understanding of percutaneous interventional procedures and training in the use of the endoarterial biopsy catheter.
DISCLAIMER: The material provided on this site is strictly for informational purposes. Do not use this site as a substitute for medical care or medical advice. Please consult with your physician or other medical care provider regarding any medical questions you may have.