Lung Transplant Rejection
The endoarterial biopsy catheter will improve the medical management of organ transplant patients, beginning with lung transplant recipients where there is a large unmet need. Compared to heart, kidney, and liver transplants, patients who receive donated lungs face the worst five-year survival rate.
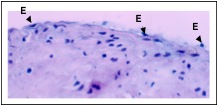 Endoarterial biopsy sample during “early rejection” showing minimal reactive endothelial cell (E) changes, with rounded, plump nuclei.
Endoarterial biopsy sample during “early rejection” showing minimal reactive endothelial cell (E) changes, with rounded, plump nuclei.
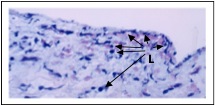 Subendothelial and medial inflammation consistent with “late rejection in an endoarterial biopsy sample.” Numerous lymphocytes (L) are present immediately under the endothelium and within the smooth muscle layer.
Subendothelial and medial inflammation consistent with “late rejection in an endoarterial biopsy sample.” Numerous lymphocytes (L) are present immediately under the endothelium and within the smooth muscle layer.
Life for a lung transplant patient involves balancing the need to suppress the immune system’s rejection of the donated lungs, and the opposite need to fight off infections during immunosuppression. Physicians providing follow-up treatment for lung transplant patients struggle to determine whether a patient is experiencing symptoms of infection or rejection –as both conditions appear outwardly the same. Even x-rays and blood tests are non-specific.
Because of the low yield of the diagnostic tests, the current treatment method is to address both conditions simultaneously. The shortcomings of this approach are obvious. The patient’s immune system is being suppressed when an infection may be present (weakening the body’s natural defenses), and the patient is being administered high doses of powerful antibiotics that would be unnecessary if only rejection is occurring (and will eventually cause the forced evolution of antibiotic resistant infections). The endoarterial biopsy catheter has the potential to solve this dilemma by more definitively resolving the infection versus rejection question through the examination of arterial biopsies from the transplanted organ.
Obtaining an endoarterial biopsy sample would more clearly indicate the presence or absence of rejection and thus allow the optimal therapy to be given. We have demonstrated the feasibility of endoarterial biopsy in animal models of transplant rejection. In addition to feasibility and pre-clinical safety, our studies demonstrated that transplant rejection can not only be detected by endoarterial biopsy – it can also be anticipated, and thus avoided. The endoarterial biopsy catheter has the potential to be used on a surveillance basis for organ transplant recipients, by detecting the earliest proteomic signs of vascular changes associated with acute and chronic rejection (also known as bronchiolitis obliterans or BOS). Physicians can prevent acute and chronic rejection by adjusting immunosuppression levels before irreversible changes occur in the targeted transplanted organs. Also, overmedication can be minimized if no serious vascular changes are found in the arterial biopsies. It has been demonstrated in the scientific literature that vascular changes are some of the earliest and most sensitive changes associated with transplant rejection and other diseases.
WARNING: Investigational Device. Limited by Federal law to investigational use. This device should be used only by physicians with a thorough understanding of percutaneous interventional procedures and training in the use of the endoarterial biopsy catheter.
DISCLAIMER: The material provided on this site is strictly for informational purposes. Do not use this site as a substitute for medical care or medical advice. Please consult with your physician or other medical care provider regarding any medical questions you may have.

