Product Pipeline
At Vascular Biosciences, we focus our research and development on seriously ill patients with unmet medical needs. Through major research efforts across multiple product lines — including targeted pharmaceuticals, interventional devices, and molecular diagnostics — VBS is developing solutions for serious diseases. We are dedicated to delivering innovative biomedical technologies that will benefit patients in need around the world.
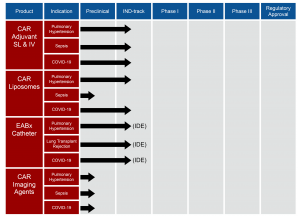
Vascular BioSciences was voted by its industry peers as having the most promising pipeline in the biotechnology industry. Our current product pipeline is divided into four areas: CAR Adjuvant, CAR Liposomes, Endoarterial Biopsy (EABx) Catheter, and targeted Imaging Agents.
CAR peptide is a 9-amino-acid cyclic peptide that selectively targets and penetrates diseased tissues, while augmenting the efficacy of therapies. Indications of pulmonary hypertension, sepsis, gene therapy, kidney disease, cachexia and COVID-19, could prompt the use of CAR adjuvant. Our lead indications for CAR peptide are pulmonary hypertension for chronic use and COVID-19 and sepsis for acute treatment.
VBS is also developing a targetable and inhalable formulation of the two drugs fasudil and DETA NONOate (DN) — a rho-kinase inhibitor and a nitric oxide (NO) donor, respectively — as a novel treatment for pulmonary hypertension. This combination therapy will encapsulate both drugs in liposomes modified with CAR peptide, to accumulate preferentially in hypertensive pulmonary arteries. In a series of preclinical studies, collaborators have demonstrated that CAR-modified liposomes containing fasudil and DN reduce the mean pulmonary arterial pressure (mPAP), which ameliorates various features of pulmonary arterial remodeling. Our CAR liposomes are currently on the IND track, and clinical translation of this combination therapy will also establish CAR-liposomes of fasudil-plus-DN as a novel and inhalable therapeutic option for pulmonary hypertension patients that will: a) specifically target the hypertensive pulmonary vasculature, and b) provide synergistic therapeutic benefits through both the Rho A/Rho kinase and NO donor pathways, without the additive adverse side effect of systemic vasodilation. CAR liposomes can also be used to deliver inhaled and intravenous COVID-19 therapeutics.
Vascular BioSciences received a Humanitarian Use Device (HUD) designation from the FDA for its Endoarterial Biopsy (EABx) Catheter to take pulmonary artery biopsies in patients who have Group 1 pulmonary arterial hypertension. The HUD designation establishes the EABx Catheter as a medical device intended to benefit patients in the treatment or diagnosis of a rare disease. We are currently working on submitting an application for a Humanitarian Device Exemption (HDE) approval from the FDA for the use of the EABx Catheter in Group 1 pulmonary arterial hypertension patients. The EABx Catheter has reached the IDE track, and we intend to launch First-In-Human clinical trials later this year.
VBS is also developing a targeted treatment to decrease the mortality rate of COVID-19 patients. A recent study that analyzed COVID-19 patients that were hospitalized showed that 42% of survivors and 100% of non-survivors developed sepsis as part of the disease progression1. In previous experiments, treatment of CAR + low dose corticosteroids in LPS septic rats increased the 48 hour survival rate from 21% to 90% compared to untreated LPS mice. This survival rate was higher than LPS rats treated with corticosteroids alone at the same dose (30%). Similar results were observed in the 2-CLP sepsis model as CAR + low dose corticosteroid treatment increased 40 hour survival from 0% in untreated and HCT treated groups to 80%. Currently, the FDA recommends the use of dexamethasone, a corticosteroid, in COVID-19 patients who are mechanically ventilated. VBS is fast-tracking to add CAR to the current low dose steroids standard of care. The purpose of adding CAR to dexamethasone treatment is to increase the survival rate of COVID-19 patients.
1. Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 (2020).
Next Steps:
- Learn about the CAR peptide
- Learn about the endoarterial biopsy (EABx) catheter
- View our products & services
- For inquiries about our product pipeline and other VBS products & services,
please contact us at pipeline@vascularbiosciences.com

